Background
Historical perspective on policies
The struggle against NTDs has substantially transformed over the past century. In the late 1970s, Kenneth S. Warren, a prominent medical researcher and director of Health at the Rockefeller Foundation, initiated a focused inquiry into immunoparasitology. His primary objective was to develop a cost-effective therapeutic intervention for the infectious diseases that disproportionately impacted populations in developing nations (Keating, 2014).
As a researcher committed to establishing a network of laboratories for studying parasitic diseases, Warren referred to them as the ‘great neglected diseases (GND) of mankind’ to encourage scientists to research these ‘overlooked’ diseases. Paralleling with this initiative, in 1976, the WHO established the Special Programme for Research and Training in Tropical Diseases (TDR) to support research in the fight against diseases of poverty (Adetokunho, 1977). These grassroots efforts laid the groundwork for the development of targeted disease-specific programs that emerged in the following years.
Disease-specific programs
In the late 1990s, the WHO launched the Roll Back Malaria (RBM) initiative to reduce malaria mortality, improve health facilities, and elevate malaria control on the global political agenda. Its main strategies included: establishing partnerships with pharmaceutical companies to lower the cost of combination therapies; monitoring antimalarial drug resistance using WHO methodologies, as outlined in the Method for Surveillance of Antimalarial Drug Efficacy (WHO, 2009); promoting environmentally sound mosquito-control alternatives in collaboration with the United Nations Environment Programme (UNEP); and forming R&D partnerships, such as with the Fogarty International Centre of the U.S. National Institutes of Health to provide research training in Africa, the Center for International Development at Harvard University to improve economic studies on the disease, and Medicines for Malaria Venture (MMV), a PPP dedicated to the discovery and development of new antimalarial drugs.
The program, with particular emphasis on sub-Saharan African countries, has successfully helped avoid approximately 6 million deaths from malaria (WHO, 1999). Nonetheless, the World Malaria Report 2024 reported that in 2023 there were 263 million new cases of malaria, with 94% of these cases occurring in African nations. Despite the significant progress, the prevalence of malaria remains inextricably linked to socio-economic factors, particularly poverty, and disproportionately affects vulnerable demographics, notably children and women (WHO, 2024).
Partnerships
In December 2003, under the auspices of Warren’s significant contributions, the WHO, and the Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ) convened the inaugural Berlin conference to discuss strategic approaches towards tropical diseases. The terminology initially established by Warren fell into disuse as Professors Fenwick,
Molyneux, and Hotez introduced the more widely accepted term ‘neglected tropical diseases’ (Molyneux, 2012; Molyneux et al., 2021).
To elevate global awareness regarding NTDs, the Sabin Vaccine Institute, in collaboration with WHO, established the Global Network for Neglected Tropical Diseases (GNNTD) in 2006 (Molyneux et al., 2021). Committed to fostering increased awareness, enhancing political commitment, and mobilizing financial resources essential for the effective control of the most prevalent NTDs, this non-profit contributed significantly to the WHO NTDs Roadmap for 2030 and received $34 million from the Bill & Melinda Gates Foundation to keep their goals and expand their range.
Furthermore, as a strategic initiative to enhance efforts, such as vector control programs and improved diagnostic access, the Uniting to Combat Neglected Tropical Diseases coalition actively collaborates with a diverse array of 150 collaborators, including governmental institutions, pharmaceutical corporations, and academic associations. Following the London Declaration and with the support of this coalition, the President of the Republic of Rwanda H.E. Paul Kagame, created the Kigali Declaration on Neglected Tropical Diseases. This declaration seeks to galvanize the involvement of individuals, communities, and nations, positioning them at the forefront of the global response to NTDs (Uniting to Combat Neglected Tropical Diseases, 2022).
Exclusion from Millennium Development Goals (MDGs)
Global health initiatives in the early 2000s were largely focused on the “Big Three” — HIV/AIDS, tuberculosis, and malaria — due to their widespread mortality during the 20th century (Makam & Matsa, 2021). These diseases became a priority for the Millennium Development Goals (MDGs).
However, this intense attention led to the omission of NTDs from the MDGs, which continued to have a severe impact on impoverished and marginalized communities. Nevertheless, this exclusion functioned as a catalyst to advocate for more attention towards NTDs in the global health scenario (Smith & Taylor, 2013). Over time, increasing recognition of their burden led to a major policy shift—NTDs, once absent from the MDGs, became a key priority within the Sustainable Development Goals (SDGs) (Malecela, 2019).
Global integration
In the context of the 2030 Agenda, the international community established the SDGs, a comprehensive framework comprising 17 objectives aimed at fostering prosperity while ensuring the protection of the planet. This initiative was launched at the United Nations Conference on Sustainable Development held in Rio de Janeiro, Brazil, in 2012.
As a response to the increasing incidence of NTDs and growing global attention towards these health challenges, member countries introduced SDG 3.3 as part of the United Nations' 2030 Agenda for Sustainable Development in 2015. This specific target, entitled “End the transmission of communicable diseases such as HIV, malaria, tuberculosis, and neglected diseases,” represents a significant advancement in efforts to reduce health disparities, enhance the overall quality of life, and stabilize the global economy (United Nations [UN], 2015; Pan American Health Organization [PAHO], 2022).
Global policy landscape
WHO Roadmap
Despite the efforts to fulfill the London Declaration’s goal of eliminating NTDs by 2020, the partnerships have not yet achieved this objective. Accordingly, a revised and more comprehensive strategy became necessary, leading to the elaboration of the WHO Roadmap (2021-2030), which outlines new approaches and targets for NTDs (WHO, 2021). The roadmap functions as both a guideline document for partnerships and prospective programs addressing NTDs, and a policy document that underscores critical challenges existing initiatives face. It identified four principal gaps that impede progress in the battle against NTDs (Souza et al., 2021; Malecela & Ducker, 2021):
- Diagnosis
- Monitoring and evaluation
- Access and logistics
- Advocacy and funding
Diagnosis
Figure 1
Assessment of diagnostic gaps and priorities for neglected tropical diseases

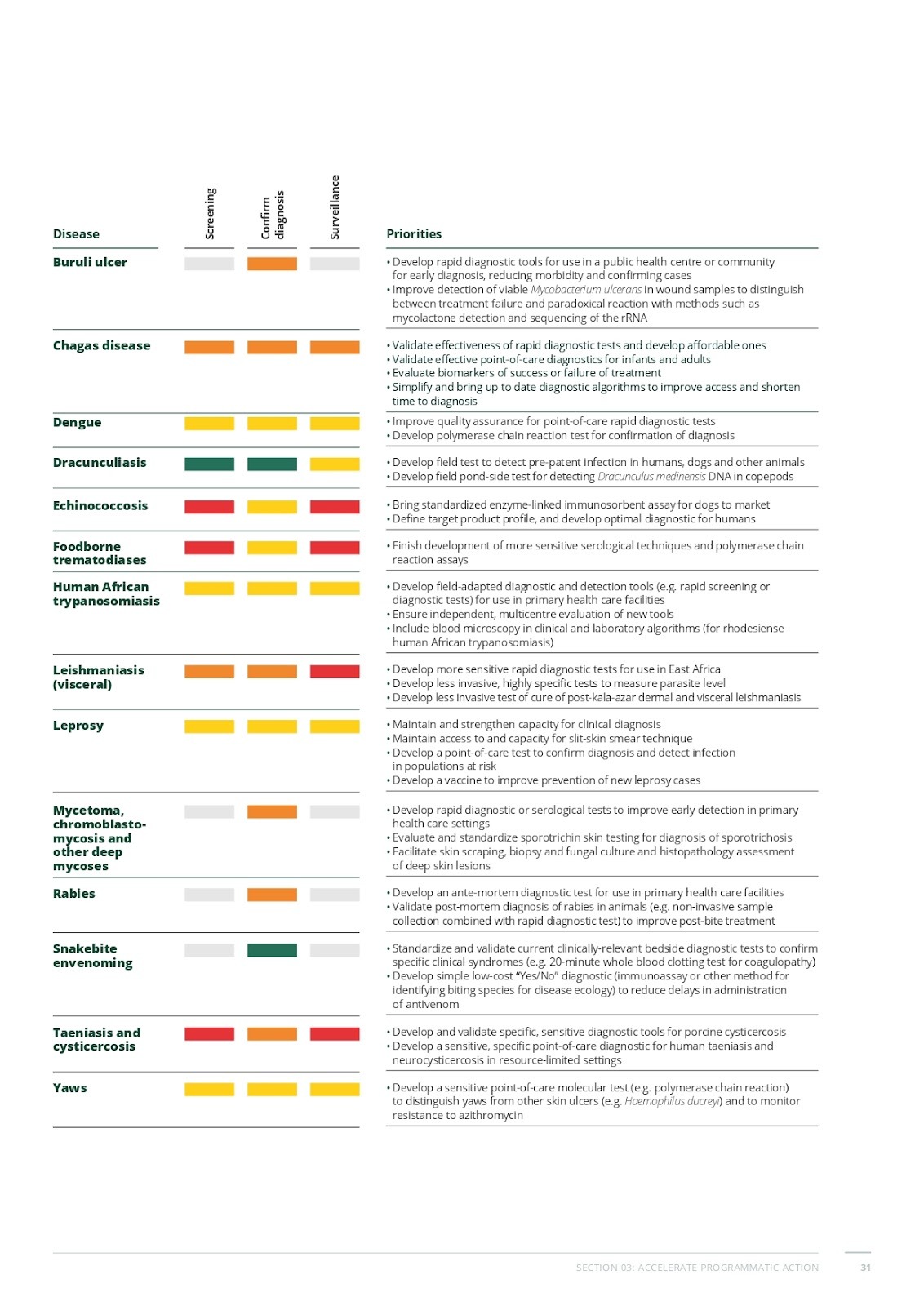
(World Health Organization, 2021)
As a way to optimize programs’ focus, the roadmap created a table categorizing priorities for different NTDs based on key intervention states: mapping, starting treatment, scaling treatment, stopping treatment, and post-treatment surveillance. The images above display a color-coded bar highlighting the stages where significant challenges arise. The rightmost column outlines the priorities for enhancing diagnostics, treatment strategies, and disease monitoring. This organized approach helps identify critical gaps and direct targeted interventions for each NTD.
Monitoring and evaluation
The roadmap indicated that the Secretariat assists member States by providing integrated data systems to enhance the collection and reporting of disease-related information. Moreover, in collaboration with the Food and Agriculture Organization (FAO), WHO has developed disease-specific documents that outline epidemiological trends, helping health ministries and non-governmental institutions monitor the impact of their activities (WHO, 2021). Since monitoring data at all levels is crucial for reaching the goals towards 2030, the roadmap established five core components of monitoring and evaluation:
- Data management platforms: comprehensive data systems that include information about the age, gender, and location of the infected individuals to facilitate decision-making.
- Data and analytics tools: data systems must contain information to improve interpretation, such as weather, patterns of land, and socioeconomic factors.
- Mapping and impact assessments: data should be adaptable, combinable, and shareable across programs.
- Surveillance: routine systems need new tools for post-validation surveillance, drug efficacy monitoring, and resistance tracking, especially as diseases are eliminated.
- Reporting: national authorities are responsible for creating an integrated reporting system for tracking progress, ensuring coordination, and improving program delivery now and in the future.
Access and logistics
To accomplish the roadmap’s goals, the establishment of an effective supply chain is essential. Ensuring the timely and adequate delivery of medicine and other health-related products requires the implementation of efficient strategies from research and development to supply chain management, quality assurance, regulatory registration, pricing mechanisms, and rational utilization. A comprehensive approach in these areas is critical for optimizing health outcomes and enhancing the accessibility of essential health commodities. To address the main challenges faced along the NTDs supply chain, the roadmap divided them into forecasting and procurement, transport and receipt at port, health ministry and warehouses, distribution, and inventory management at health facilities, emphasizing the major hindrances that slow the process of securing access to safe, effective, and affordable health products.
Advocacy and funding
The United States Agency for International Development estimates that for every US$ 1 spent, US$ 26 worth of donated medicine is delivered through collaborations with pharmaceutical companies (Uniting to Combat Neglected Tropical Diseases, 2025). Consequently, NTDs treatments are often regarded as among the “best buys” in the realm of international development, given their capacity to yield significant social and financial returns while remaining cost-effective.
Regarding preventive chemotherapy, which entails the systematic and large-scale administration of drugs, it is projected that for each dollar invested, a return of US$25 can be realized — reflecting an annualized return rate of 30% (Montresor et al., 2012; Fitzpatrick, 2018).
Despite the considerable mobilization of around three billion dollars through the London Declaration, additional funding is still required for certain diseases, especially those nearing elimination. As an example of this situation, in 2016, the WHO Alliance for the Global Elimination of Trachoma by 2020 calculated that the cost of the elimination of trachoma would amount to approximately US$ 1 billion; however, only US$ 200-300 million had been pledged at that time (WHO, 2016). Thus, NTDs must be integrated into national health strategies and budgets rather than treated as isolated programs. Such integration could be achieved at a cost of as little as 1% of domestic expenditure on health to meet the goals set for 2030.
Global burden and integration into SDGs
NTDs cause chronic suffering, disability, and economic adversity, disproportionately affecting marginalized and impoverished communities. A metric called the Disability-Adjusted Life Year (DALY) is used to measure the disease burden and is calculated with the following formula:
DALY = YLL + YLD
where:
- YYL (Years of Life Lost) represents premature mortality.
- YLD (Years of Life Lived with Disability) represents the burden of disease-related disability.
Figure 2 shows the global burden of NTDs around the world. Their significance in the Southern Hemisphere is notable, with predominant cases in Brazil, Central and East Africa, followed by some countries in East Asia . Correspondingly, Figure 3 illustrates the consequences of these cases. The World Bank, in 2017, estimated that NTDs had a total loss of more than 25 million DALYs, with scabies and foodborne trematode infections sharing the highest numbers.
Figure 2
Global burden of NTDs, showing the number of people requiring interventions per country
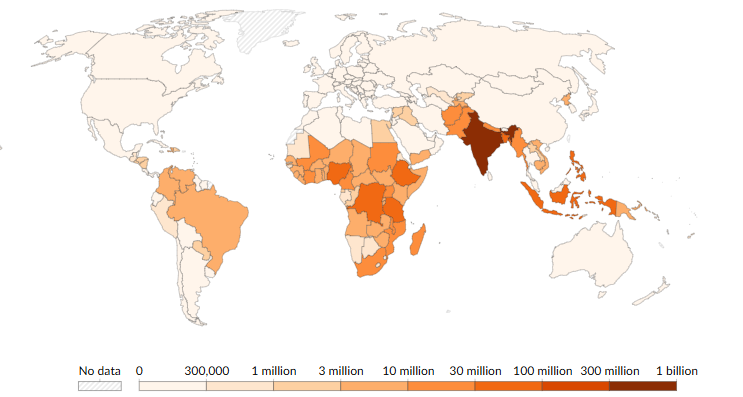
(WHO — Global Health Observatory, 2023)
Figure 3
Global burden of neglected tropical diseases (NTDs) by region in 2017, measured in DALYs.
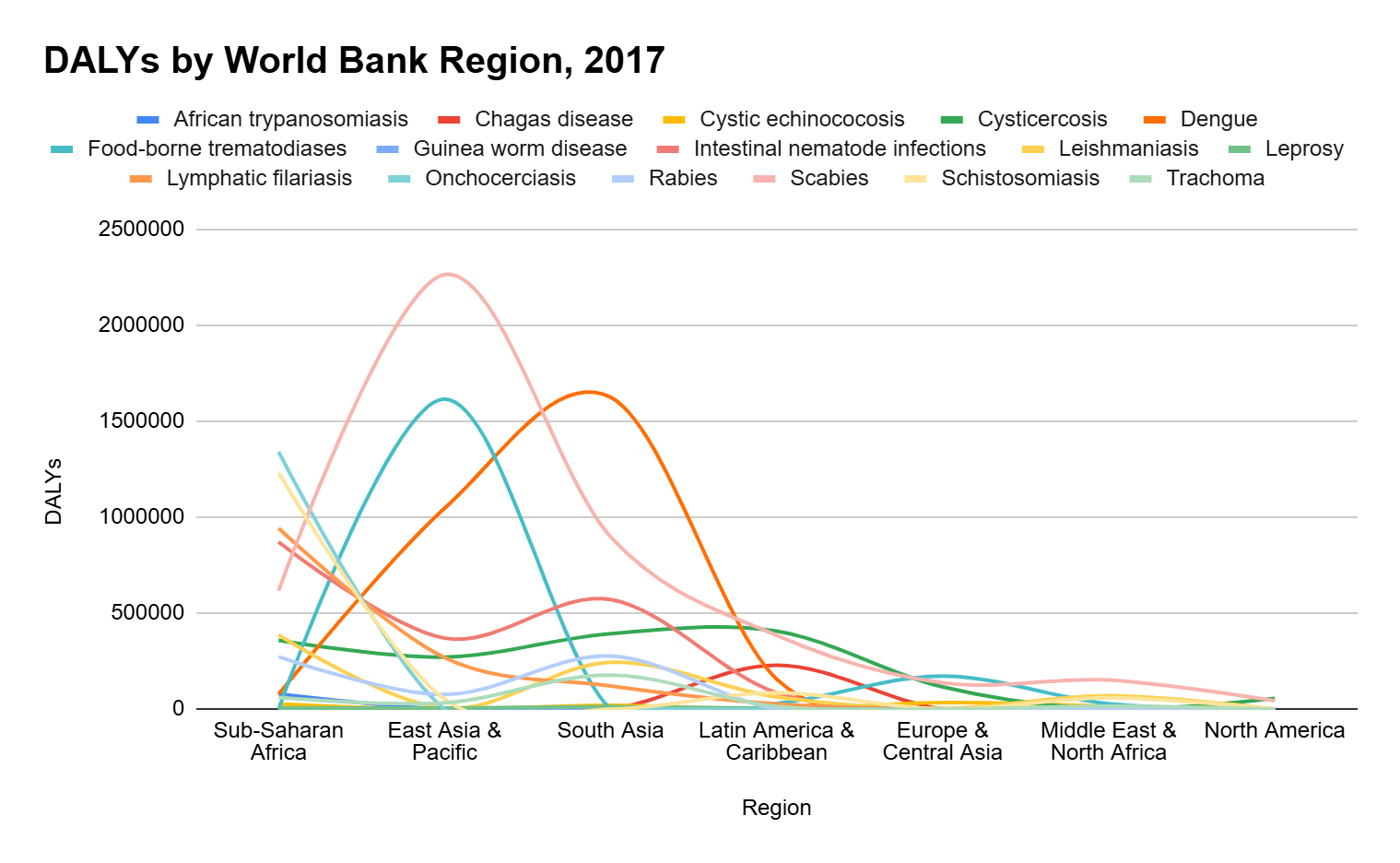
(Adapted from Japan Alliance on Global Neglected Tropical Diseases, 2017)
Given the substantial burden of NTDs reflected in DALY estimates, it became clear that targeted action was necessary to mitigate their impact. In response, WHO pushed their inclusion by introducing a target, under SDG 3 (Good Health and Well Being), on communicable diseases. Expressing the goal of ending the epidemic of AIDS, tuberculosis, malaria, and neglected tropical diseases, as well as combating hepatitis, water-borne diseases, and other communicable diseases by 2030.
The inclusion of NTDs in SDG 3.3 reflects a response to their historical neglect, the scarcity of funding, and the insufficient integration of these diseases into national healthcare systems and programs (Vanderslott, 2019). The incorporation of NTDs has expanded the discussion of the “Big Three” into the “gang of four” (Smith & Taylor, 2013). The WHO has acknowledged that without specific international targets, the advancement in the control and elimination of these diseases will remain slow. Moreover, emerging interventions, such as mass drug administration (MDA) and improved diagnostic tools, have underscored that the elimination of certain NTDs is not only feasible but also financially beneficial. These initiatives can lead to significant cost savings by reducing the need for long-term treatment and management of these diseases,
as well as improving productivity and quality of life, reducing healthcare costs, and increasing economic growth in affected regions (Keenan et al., 2013).
National and regional approaches
Case studies
Guinea Worm Eradication Program
Guinea worm disease (or dracunculiasis) is a parasitic disease currently limited to rural and remote areas of Sub-Saharan countries, as shown in Figure 4, that lack access to basic sanitation. The great majority of its cases are during agricultural peaks, leading to more sick people, a loss in productivity, and, consequently, an economic loss for the region (Greenaway, 2004).
Figure 4
Map of current and former dracunculiasis-endemic countries
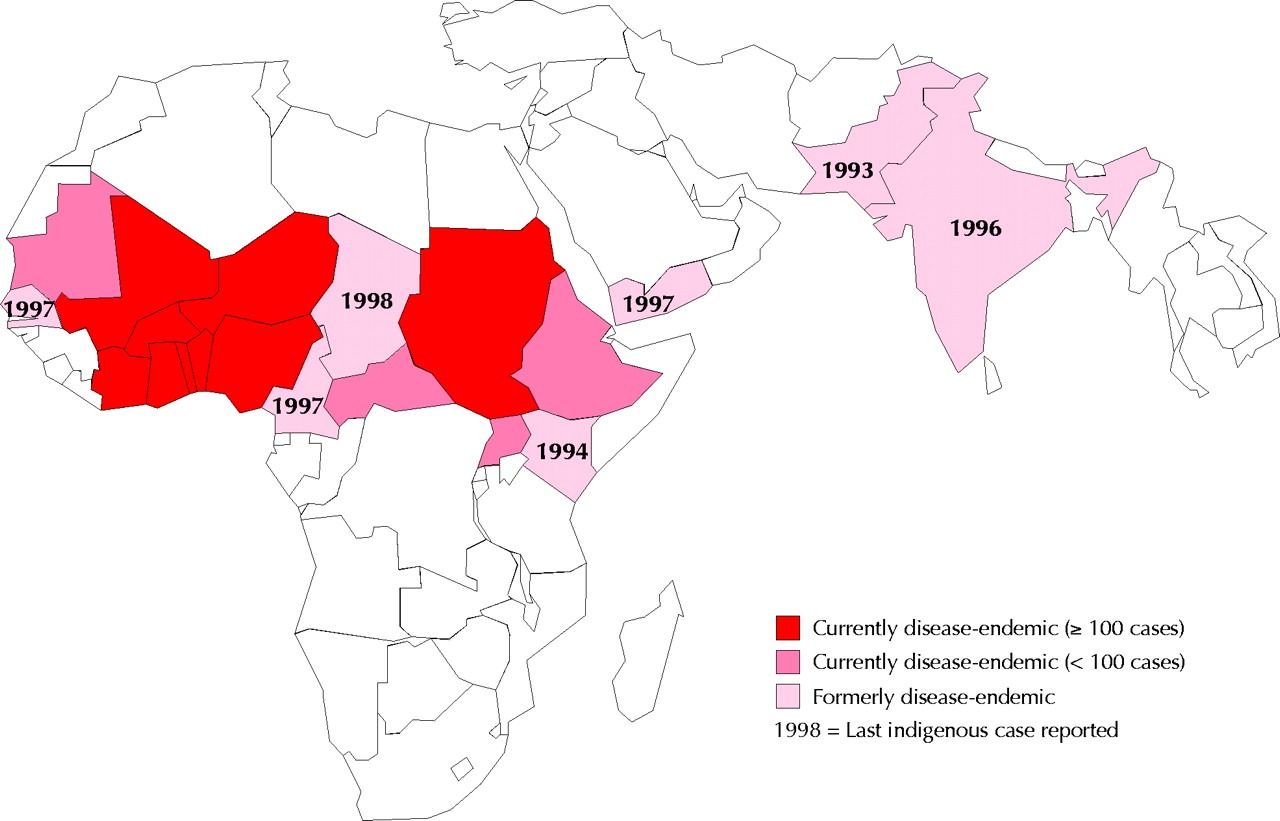
(Greenaway, 2004)
Although rarely lethal, during the mid-1980s, there were approximately 3.5 million cases of guinea worm disease, calling the international community’s attention to the issue (WHO, 2022a). Thus, the Carter Center, a nongovernmental organization committed to human rights, along with health ministries, the U.S. Centers for Disease Control and Prevention, the WHO, and the United Nations Children's Fund (UNICEF), initiated an international campaign to eradicate the disease. Since there was no treatment, their efforts focused on stopping the spread of guinea worms by providing health education, such as teaching local people how to filter their water and to keep anyone with an emerging worm from entering water sources.
In Chad, for instance, one significant challenge was the large number of cases involving domesticated animals. To address this issue, the Carter Center, in collaboration with volunteers trained by the Chad’s Guinea Worm Eradication Program (GWEP), implemented educational initiatives aimed at guiding the local population. The strategies included burning of fish discards and cash incentives for those who reported cases of animals and kept them far from water sources.
Before having been certified as free from dracunculiasis by WHO in 2000, India launched their National GWEP in 1983-84 (Kumar, 2000). Unlike Chad, India successfully eliminated the disease in its territory, having its last case reported in July 1996. GWEP, alongside WHO, strategies based on the worm’s life cycle and modes of transmission included: vector control through the use of the chemical compound Temephos (ABATE®) eight times a year in unsafe water sources, provision of clear water in at-risk villages, and health education for local communities.
At the end of 2016, 17 of the 21 endemic countries ended the transmission, and 15 had the elimination of the disease certified by WHO (Molyneux & Sankara, 2017). From the beginning of the program in 1986 to 2024, the number of cases dropped from 3.5 million to 14 worldwide. Despite considerable success, five endemic countries remain: Angola, Chad, Ethiopia, Mali, and South Sudan. Although the case of human numbers is considerably low, since 2000, there has been an increase in the number of cases in animals, such as dogs, cats, and baboons. In 2023, there were 14 human cases and 886 animal cases (Hopkins, 2024). Researchers hypothesize that animals that eat more fish have a higher chance of getting infected, and that is because fish and fish guts might be a novel route for transmission (McDonald et al., 2020). Currently, the most sustainable and reliable method of stopping the transmission is to abate the infected animals (Elom, 2020).
Onchocerciasis Control Programme and African Programme for Onchocerciasis Control
Onchocerciasis (or river blindness) is a parasitic disease predominantly endemic in Africa and the Arabian Peninsula (Figure 5), although about 1% of cases occur in Latin America (Schmidt et al., 2022; WHO, 2022b). Currently, it affects more than 37 million people worldwide, and although it has never caused a single death, it is responsible for a global burden of 987,000 DALYs (Sighsavers, 2017).
Figure 5
Distribution of onchocerciasis in Latin America, Africa, and the Arabian Peninsula
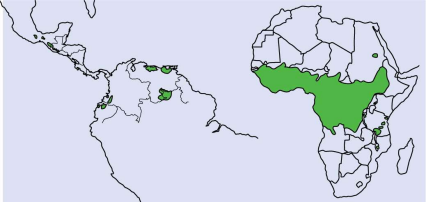
(Burnham, 1998)
Given the significant disease burden and its global impact on affected communities, the Onchocerciasis Control Programme (OCP) was launched in 1974 to combat the disease in West Africa through large-scale vector control, primarily aerial larviciding of blackfly breeding sites. A policy-driven, intergovernmental partnership coordinated by the WHO, supported by UN Development Programme (UNDP) and FAO, and funded by donor countries and agencies through a trust fund administered by the World Bank, establishing a centralized governance model that enabled coordination of vector control operations across international borders (World Bank, 1984). Through this institutional backing, it was possible to control the vector using helicopters and aircraft at high velocity to spray larvicidal pesticides over the breeding sites of blackflies (Onifade et al., 2024). Covering an area of more than 650,000 km², this strategy played a major role in mitigating the disease (Boatin, 2008).
The OCP successfully reduced the transmission of onchocerciasis in West Africa, significantly lowering infection rates and preventing blindness in millions. However, onchocerciasis remained a major health concern in other parts of Africa, particularly in Central and East Africa, where OCP had not been implemented.
Thus, the WHO launched the African Programme for Onchocerciasis Control (APOC) in 1995, aiming to eliminate onchocerciasis in Central and East Africa through the organization of MDA through a decentralized, community-led policy model. Its core strategy was established through a collaborative effort involving the WHO, UNICEF, UNDP, and TDR, a novel approach named Community-Directed Treatment with Ivermectin (CDTi) was developed (Moshi, 2021). The donation of the drug was made feasible through the formally instituted Mectizan Donation Program established by the pharmaceutical company Merck. Additionally, with the World Bank serving as an intermediary, the coordination of the program was founded by donor countries and national onchocerciasis task forces, such as beneficiary governments and non-governmental development organizations (Coffeng et al., 2013).
The MDA strategy empowered, and still empowers, communities in affected regions to assume responsibility for the organization and implementation of their drug distribution. The partnerships established helped CDTi coverage from 1.5 million people in 1997 to over 112 million in 2012 (WHO, 2015).
Figure 6
A map of Africa, showing the areas covered by the African Programme for Onchocerciasis Control in green and the African Programme Onchocerciasis Control in yellow
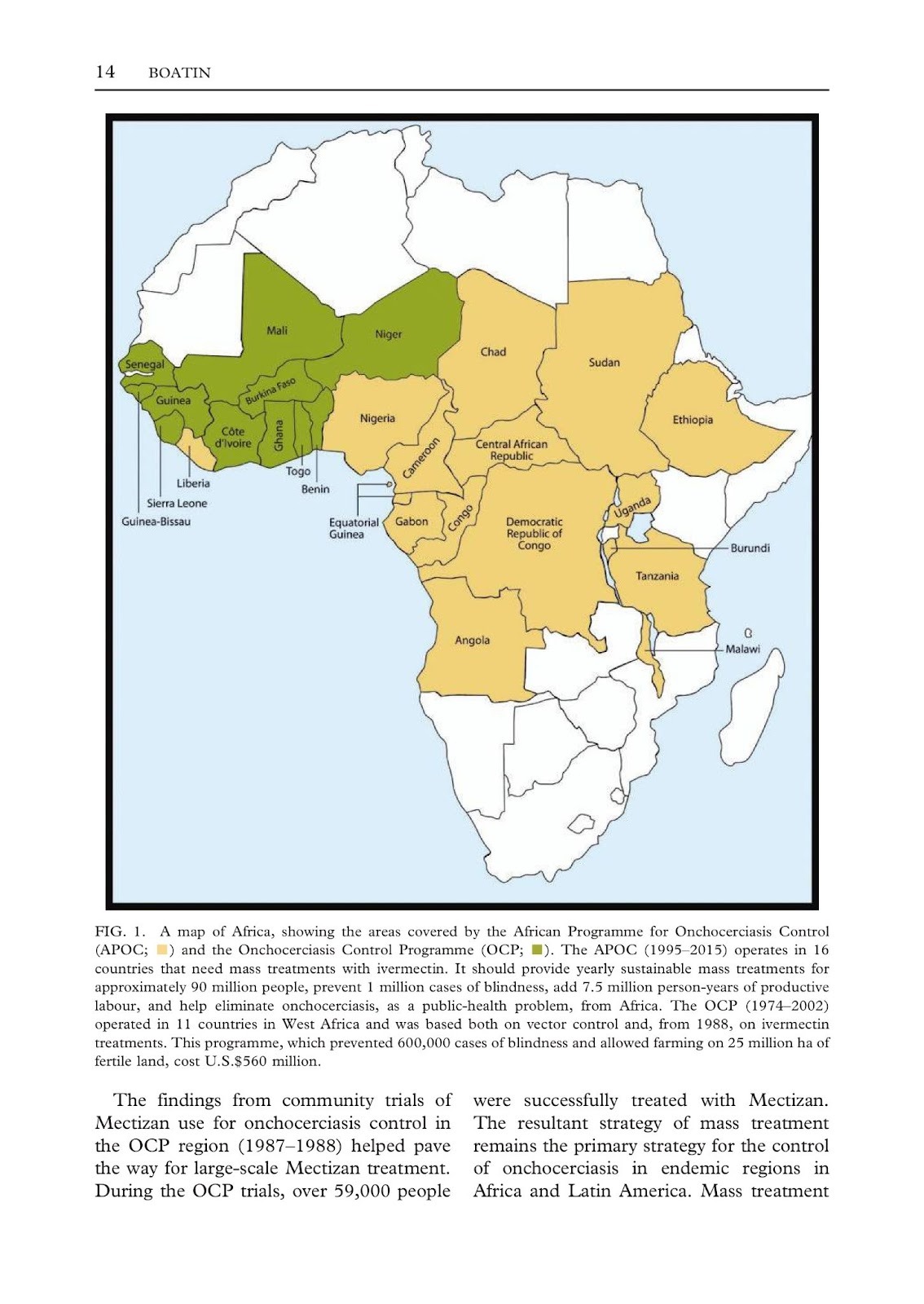
(Boatin, 2008)
Together, OCP and APOC covered 27 countries on the African continent. OCP operated from 1974 to 2002, treating more than 150 million people in 11 countries. Similarly, APOC was active from 1995 to 2015 and was recognized as one of the most successful programs in Africa. This program established a robust network aimed at enhancing primary healthcare systems across 16 countries, facilitating the implementation of more than 108 active projects (Brown, 2007).
Nonetheless, it is noteworthy that only in January 2025 did Niger become the first African country to eliminate onchocerciasis (Jesudason, 2025). The country’s vector control efforts were extremely important for this historical milestone. From 1976 to 1989, Niger established measures to regularly treat vector’s breeding sites with a rotation of larvicides through helicopters, which was expensive, but efficient.
Following the APOC’s discontinuation in 2015, the WHO introduced an initiative to sustain the achievements of APOC. This initiative, named Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN), extends its focus beyond onchocerciasis to encompass five PC-NTDs within the African region through MDA. Unlike its predecessors, ESPEN remains active and engages in collaboration with the WHO Regional Office for Africa (AFRO), Member States, and several NTD partners (Lakwo et al., 2020). As a result, it has successfully reached 47 countries, thereby increasing the prevalence and execution of MDA within these regions.
Financing and innovation in NTD policies
Innovative funding models
NTDs have always faced inadequate funding due to their low commercial appeal. It is estimated that only 0.6% of global healthcare funding is allocated to NTDs programs and initiatives (WHO, 2020). Traditional funding mechanisms have often been insufficient in sustaining large-scale, successful interventions.
Past initiatives have shown that several factors are mandatory for a favorable outcome (Zhang et al., 2019):
- A long-term commitment from a diverse range of donors, as well as the governments of endemic countries, is essential for tackling NTDs.
- Strong partnerships between key stakeholders, including the WHO, pharmaceutical companies, local governments, NGOs, and affected communities.
- Expanding the number of donor countries to bridge financial gaps, while collaboration among endemic nations, is key to preventing cross-border disease transmission.
- Targeted disease control efforts, alongside investments in primary healthcare systems and capacity-building initiatives, can strengthen national programs and ensure lasting impact.
For instance, the London Declaration, which lasted for eight years, mobilized 12 companies and 80 partner organizations across different fields, leading to 14 billion donated treatments and a remarkable financial contribution of 17 billion USD donated from the R&D-based biopharmaceutical industry. Such collective efforts position the program as one of the most cost-effective in the history of public health.
Public-Private Partnerships
PPPs are collaborations between governmental institutions and private companies that facilitate funding management for NTD R&D programs (Ma et al., 2023). However, most NTDs greatly impact remote areas of the world, making investments less appealing to the private sector. Nonetheless, the world has changed since the first NTDs roadmap publication in 2012, especially because of a global recognition that NTDs are not merely a local public health issue, but a global one affecting the entire tropical region (Bush & Hopkins, 2011; WHO, 2012). Therefore, PPPs are increasingly seen as an effective means to address the challenges in drug development for NTDs (Chatelain & Ioset, 2011).
Drug discovery and development (DDD) for NTDs often exceeds 10 years and costs over $1 billion (Williams et al., 2015; Weng et al., 2018). Drug innovation relies heavily on investment in R&D. Therefore, the WHO developed an “R&D Blueprint” to accelerate the control and eradication of NTDs. The document emphasizes the identification of key priorities and outlines strategic roadmaps, target product profiles (TPPs), and recommendations for the evaluation of vaccines and treatments.
In alignment with these global efforts, the Gates Foundation’s R&D funding, committed to eradicating eight of 20 NTDs, has allocated more than $1.8 billion to organizations focused on R&D for delivering innovative methods to control diseases. Ultimately, continuous and sustained investment in R&D is required to achieve significant progress in the field of DDD.
PPPs and R&D investments have played a pivotal role in addressing NTDs, fostering innovation, and improving access to treatments. One of the most successful cases is the Drugs for Neglected Tropical Diseases initiative (DNDi), which has received donations from the public and private sectors since 2003 (Ioset & Chang, 2011). The current goals and challenges faced by DNDi are entirely based on the patients’ needs. Consequently, to secure the main necessities, a pragmatic product-development partnership (PDP) approach based on a virtual model with all R&D activities outsourced has been adopted to ease their three-pronged approaches to treatment: short (1-3 years), medium (3-5 years), and long (6-15 years) (Drugs for Neglected Tropical Diseases Initiative, 2019). Additionally, to maintain a balanced distribution of contributions, no donor may provide more than 25% of the total budget (DNDi, 2014).
After more than 20 years since DNDi’s launch, the organization has developed 13 cost-effective treatments for six deadly diseases. Such initiatives include alternatives to arsenic-based medicine for sleep sickness (e.g., fexinidazole instead of melarsoprol), ravidasvir for hepatitis C, and acoziborole for sleep sickness. Ultimately, DNDi exemplifies the effectiveness of PPPs in tackling critical health challenges faced by underserved communities. By managing funding complexities and driving innovation, DNDi reinforces the importance of sustained global collaboration in the fight against neglected diseases.
Despite the major achievements of PPPs over the past couple of decades, several challenges still need to be addressed. These include a lack of transparency, accountability, and clear priorities with the public from donors (Munoz et al., 2015; Mrazek & Mossialos, 2003). In addition, these partnerships may lean toward altering existing medicines for the market rather than creating new and more effective ones for many reasons (Bors et al., 2015). PPPs’ past results have shown more effectiveness in improving current medications than when creating new ones, as observed in MMV, a PPP focused on malaria treatments, which had more success in altering the formula of the already existing medicine Coartem™ into a chewable tablet. Lack of market incentives, the high cost and time required for new drug development, and inconsistent and unpredictable financing are additional reasons for this trend (Bathurst & Hentschel, 2006).
Non-governmental development organizations
Since the 1980s, a growing number of non-profit civic organizations have emerged as alternatives to support the governments of developing countries (Martín Nieto, 2007; Fowler, 2000). To differentiate these institutions from the ones associated with the State, the United Nations (UN) labeled them as non-governmental development organizations (NGDOs).
Within the global health landscape, NGDOs have become instrumental in tackling NTDs, especially through the NTD NGDO Network. These organizations have fully committed to the London Declaration during the Network’s third annual meeting in Australia in 2012. Thus, a few of their commitments include:
- The integration of water, sanitation, and hygiene (WASH) programs into NTD programs where appropriate.
- The development of clear guidelines as to where and when to stop treatment.
- The finalization of baseline disease mapping to achieve the scale-up of treatment.
By aligning these commitments with broader initiatives, NGDOs have played a crucial role as major innovators for community-wide treatment and have distributed millions of treatments of PCs over the past decade (Bush & Hopkins, 2011).
Mass drug administration
MDA is a strategy in which an entire population is subjected to treatment without individual diagnosis. Highly recommended by WHO, MDA offers treatment against NTDs to more than 700 million people annually. It is estimated that, with pharmaceutical partners’ donations, it is possible to treat each person for only fifty cents per year (UN, 2009).
Each drug has a specific dosing requirement and must be distributed according to the patient’s weight. Ivermectin, for instance, is the only reliable drug for onchocerciasis through MDA. It is distributed at a standard dose of 150µg/kg (Campillo et al., 2020). Praziquantel, on the other hand, is distributed at a single and standard dose of 40 mg/kg, meaning the standard amount is delivered in a single administration, and is currently the only drug available to treat schistosomiasis (Olliaro et al., 2013). Azithromycin, however, used to treat Chlamydia trachomatis eye infection, is distributed at a single and standard dose of 20 mg/kg orally (Cochereau et al., 2007).
While MDA has proven to be a cost-effective strategy in reducing the burden of NTDs, its success depends on several factors. Papers on lymphatic filariasis (LF) analyzed key challenges faced during MDA distribution to control the disease in Ghana. While the entire territory was covered, there were many obstacles faced, such as the population’s non-compliance with treatment (some of them discarded the drugs to avoid side effects), weak supervision of volunteers responsible for distributing the drugs, and unreliable data (Ahorlu et al., 2018; Anto et al., 2011; Manyeh et al., 2020; de Souza et al., 2016). Therefore, these challenges understate the importance of strengthening community engagement, ensuring proper training for treatment distributors, and improving data collection methods.
Policy challenges and gaps
Weak healthcare infrastructure in endemic regions
Alongside inadequate funding, weak infrastructure is one of the main reasons that NTDs continue to exist (Qian et al., 2020). Inaccessibility of clean water and sanitation contributes to the continuous life cycle of several highly infectious parasites, such as the ones that cause soil-transmitted helminths (STHs), responsible for infecting more than one and a half billion people worldwide (Hotez et al., 2011; Chen et al., 2024). According to the Progress on Drinking Water, Sanitation and Hygiene 2000–2017 report from UNICEF, rural areas are the most affected. Eight out of ten people lack clean water, and seven out of ten lack basic sanitation, living in rural areas (WHO, 2019).
A study developed by the Economics Department of the Federal University of Minas Gerais (UFMG) analyzing municipalities from the North and Northeast regions of Brazil stated that, in some areas, the shortest distance from a city to a hospital with an ICU bed can reach almost 400 kilometers (Pinto, 2019). These findings highlight a critical healthcare issue and underscore an urgent need for target investments in healthcare infrastructure.
These factors are intrinsically related to poverty, creating a cycle: lack of clean water, sanitation, and basic healthcare fuels the spread of infectious diseases, reducing individuals’ ability to work, attend school, and maintain productivity. Thus, deepening economic hardship.
The goals of universal access to safe drinking water and sanitation can only be reached through a collaborative effort involving national governments in conjunction with communities, civil society organizations, the private sector, and international development agencies. Governmental entities must formulate and execute policies that ensure the provision of clean water and sanitation, and improved access to healthcare facilities, such as hospitals and health posts, which are community-level clinics that provide basic medical services.
Climate change and its impact on NTD spread
The World Population Prospects 2024 report projects that, by 2100, the world’s population will exceed ten billion (DESA/Population Division, 2024). However, since the beginning of the Industrial Revolution, the exploitation of natural materials has become increasingly common. Consequently, from 1760 to 2019, carbon dioxide levels in the atmosphere rose by almost 50% (Zhang et al., 2019). Therefore, historical studies have substantiated the basic principle of climate change: increasing greenhouse gas emissions drives the global mean temperature upward (Booth, 2018). It is estimated that, by the end of the 21st century, the average global temperature will rise by 1.9°C (Change, 2007).
Elevated temperatures, rainfall, humidity, and increased sea levels are among the major causes of geographical shifts in vector distribution and disease transmission (Tidman, Abela-Ridder, and De Castañeda, 2021; Chen et al., 2011). Mosquitoes that transmit dengue fever, such as Aedes aegypti and Aedes albopictus, predominantly thrive in environments with elevated temperatures and high humidity. Consequently, projections indicate that these conditions could expose an additional 500 million individuals to chikungunya and dengue, with estimates suggesting that this number could approach one billion by 2080 (Ryan et al., 2019). Additionally, climatic hazards bring people closer to pathogens. Heatwaves, for instance, increase water-related activities, thereby heightening the risk of transmitting waterborne diseases (Phillips, 2024).
As climate change continues to accelerate, significant shifts in the geographical distribution of NTDs are expected. These diseases are likely to spread into new regions and become more severe in existing areas due to faster reproduction and development rates of parasites driven by rising temperatures (Tidman, 2021). This requires countries to move beyond simply reacting to a health crisis. Instead, they must adopt proactive policy frameworks that incorporate climate modeling, vector surveillance, and readiness within health systems. Nations in Sub-Saharan Africa, South Asia, and parts of Latin America often lack policy capacity to integrate climate projections into public health planning and remain fragile due to their vulnerable healthcare systems.
To tackle these challenges, international policy efforts must focus on equitable financing and regional cooperation. This could involve establishing programs and organizations that align health, environment, and infrastructure goals; fostering partnerships across different sectors; and establishing trust funds dedicated specifically to controlling NTDs in regions most at risk from climate change (Klepac et al., 2024).
Conclusion
Historical programs such as the OCP and APOC demonstrated how strong international cooperation, coordinated regional policies, and community-focused strategies can achieve sustainable disease management. Similarly, programs like GWEP and RBM have shown the effectiveness of targeted approaches that combine drug and intervention access, real-time monitoring, and interagency partnerships. These models highlight the importance of integrating treatment accessibility, real-time data tracking, and cooperative efforts as key components in combating diseases.
To effectively combat NTDs and meet the targets of the WHO NTD Roadmap for 2030, future policies must emphasize interventions that are both scalable and sustainable, taking into account the realities of health and climate change. This entails the incorporation of predictive climate-health modeling and the establishment of robust funding mechanisms aimed at long-term control.
Furthermore, collaboration between public and private sectors is essential to bolster community-based delivery systems, stimulate local R&D for diagnostics and treatments, and enhance cross-border cooperation to address the transnational aspects of NTD transmission. By harmonizing research, prevention, and treatment initiatives across sectors and borders, the global health community can make significant strides toward eliminating NTDs as a public health concern.
References
Adetokunho, O. L. (1977). Special Programme for Research and Training in Tropical
Diseases, TDR.
Ahorlu, C. S., Koka, E., Adu-Amankwah, S., Otchere, J., & de Souza, D. K. (2018).
Community perspectives on persistent transmission of lymphatic filariasis in three hotspot districts in Ghana after 15 rounds of mass drug administration: a qualitative assessment. BMC public health, 18, 1-10.
Anto, F., Asoala, V., Anyorigiya, T., Oduro, A., Adjuik, M., Akweongo, P., ... &
Hodgson, A. (2011). Simultaneous administration of praziquantel, ivermectin and albendazole, in a community in rural northern Ghana endemic for schistosomiasis, onchocerciasis and lymphatic filariasis. Tropical Medicine & International Health, 16(9), 1112-1119.
Bathurst, I., & Hentschel, C. (2006). Medicines for Malaria Venture: sustaining
antimalarial drug development. Trends in parasitology, 22(7), 301-307.
Boatin, B. (2008). The onchocerciasis control programme in West Africa (OCP). Annals
of Tropical Medicine & Parasitology, 102(sup1), 13-17.
Booth, M. (2018). Climate change and the neglected tropical diseases. Advances in
parasitology, 100, 39.
Bors, C., Christie, A., Gervais, D., & Wright Clayton, E. (2015). Improving access to
medicines in low‐income countries: a review of mechanisms. The Journal of World Intellectual Property, 18(1-2), 1-28.
Brown, H. (2007). Community workers key to improving Africa's primary care. The
Lancet, 370(9593), 1115-1117.
Burnham, G. (1998). Onchocerciasis. The Lancet, 351(9112), 1341-1346.
Bush, S., & Hopkins, A. D. (2011). Public–private partnerships in neglected tropical
disease control: The role of nongovernmental organisations. Acta tropica, 120,
S169-S172.
Campillo, J. T., Chesnais, C. B., Pion, S. D., Gardon, J., Kamgno, J., & Boussinesq, M.
(2020). Individuals living in an onchocerciasis focus and treated three-monthly
with ivermectin develop fewer new onchocercal nodules than individuals treated annually. Parasites & Vectors, 13, 1-11.
Carter Center Coalitions. (2022). The Carter Center.
https://www.cartercenter.org/news/publications/health/coalitions.html
Carter Center Reports 14 Human Guinea Worm Cases in 2024. (2024). The Carter
Center. https://www.cartercenter.org/news/pr/2025/2024-guinea-worm-worldwide
cases-announcement.html
Change, I. P. O. C. (2001). Climate change 2007: Impacts, adaptation and vulnerability.
Geneva, Switzerland.
Chatelain, E., & Ioset, J. R. (2011). Drug discovery and development for neglected
diseases: the DND i model. Drug design, development and therapy, 175-181.
Chen, I. C., Hill, J. K., Ohlemüller, R., Roy, D. B., & Thomas, C. D. (2011). Rapid range
shifts of species associated with high levels of climate warming. Science, 333(6045), 1024-1026.
Chen, J., Gong, Y., Chen, Q., Li, S., & Zhou, Y. (2024). Global burden of soil-transmitted
helminth infections, 1990-2021. Infectious Diseases of Poverty, 13(05), 68-77.
Cochereau, I., Goldschmidt, P., Goepogui, A., Afghani, T., Delval, L., Pouliquen, P., ... &
Robert, P. Y. (2007). Efficacy and safety of short duration azithromycin eye drops
versus azithromycin single oral dose for the treatment of trachoma in children: a
randomised, controlled, double-masked clinical trial. British journal of
ophthalmology, 91(5), 667-672.
Coffeng, L. E., Stolk, W. A., Zoure, H. G., Veerman, J. L., Agblewonu, K. B., Murdoch,
M. E., ... & Amazigo, U. V. (2013). African Programme for Onchocerciasis Control 1995–2015: model-estimated health impact and cost. PLoS neglected tropical diseases, 7(1), e2032
De Souza, D. K., Yirenkyi, E., Otchere, J., Biritwum, N. K., Ameme, D. K., Sackey, S.,
... & Wilson, M. D. (2016). Assessing lymphatic filariasis data quality in endemic communities in Ghana, using the neglected tropical diseases data quality assessment tool for preventive chemotherapy. PLoS neglected tropical diseases, 10(3), e0004590.
DESA/Population Division. (2024, July 12). World Population Prospects 2024: Summary
of Results - World. ReliefWeb. https://reliefweb.int/report/world/world population-prospects-2024-summary-results
Drugs for Neglected Diseases Initiative. (2010). Raising the Profile of Neglected Tropical
Diseases. United Nations Economic and Social Council.
Drugs for Neglected Tropical Diseases initiative. (2019). 15 Years of Needs-Driven
Innovation for Access.
Elom, M. O. (2020). Dracunculus medinensis (Guinea worm disease) elimination and
eradication and the challenges of emerging non-human animal hosts: A review of the literature. Asian J Biol Life Sci, 39-48.
Fitzpatrick, C., Nwankwo, U., Lenk, E., de Vlas, S. J., & Bundy, D. A. (2018). An
investment case for ending neglected tropical diseases.
Fowler, A. (2000). NGDOs as a moment in history: beyond aid to social entrepreneurship
or civic innovation?. Third world quarterly, 21(4), 637-654.
Government of India. (n.d.). Guinea Worm Eradication Programme – National Centre for
Disease Control (NCDC). Mohfw.gov.in. https://ncdc.mohfw.gov.in/guinea-worm-eradication-programme/
Greenaway, C. (2004). Dracunculiasis (guinea worm disease). Cmaj, 170(4), 495-500.
Hopkins, D. R. (2024). Progress toward global dracunculiasis (Guinea worm disease)
eradication, January 2023–June 2024. MMWR. Morbidity and Mortality Weekly Report, 73.
Hotez, P. J., Bundy, D. A., Beegle, K., Brooker, S., Drake, L., de Silva, N., ... & Savioli,
L. (2011). Helminth infections: soil-transmitted helminth infections and schistosomiasis.
Hotez, P. J., Alvarado, M., Basáñez, M. G., Bolliger, I., Bourne, R., Boussinesq, M., ... &
Naghavi, M. (2014). The global burden of disease study 2010: interpretation and
implications for the neglected tropical diseases. PLoS neglected tropical diseases, 8(7), e2865.
Hotez, P. J. (2021). Forgotten people, forgotten diseases: the neglected tropical diseases
and their impact on global health and development. John Wiley & Sons.
Ioset, J. R., & Chang, S. (2011). Drugs for Neglected Diseases initiative model of drug
development for neglected diseases: current status and future challenges. Future Medicinal Chemistry, 3(11), 1361-1371.
Jesudason, T. (2025). Niger eliminates onchocerciasis. The Lancet Infectious Diseases,
25(4), e204.
Kalia, N., & Kaur, M. (2018). Infectious diseases and your health. P. P. Singh (Ed.).
Springer.
Keating, C. (2014). Ken Warren and the Rockefeller Foundation’s Great Neglected
Diseases Network, 1978–1988: the transformation of tropical and global medicine. Molecular Medicine, 20, S24-S30.
Keenan, J. D., Hotez, P. J., Amza, A., Stoller, N. E., Gaynor, B. D., Porco, T. C., &
Lietman, T. M. (2013). Elimination and eradication of neglected tropical diseases with mass drug administrations: a survey of experts. PLoS neglected tropical diseases, 7(12), e2562.
Klepac, P., Hsieh, J. L., Ducker, C. L., Assoum, M., Booth, M., Byrne, I., ... & Fall, I. S.
(2024). Climate change, malaria and neglected tropical diseases: a scoping review. Transactions of The Royal Society of Tropical Medicine and Hygiene, 118(9), 561-579.
Kumar, S. (2000). Free of guineaworm, India tackles polio. The Lancet, 355(9199),
212-212.
Lakwo, T., Oguttu, D., Ukety, T., Post, R., & Bakajika, D. (2020). Onchocerciasis
elimination: progress and challenges. Research and Reports in Tropical Medicine, 81-95.
Lamb, W. F., Wiedmann, T., Pongratz, J., Andrew, R., Crippa, M., Olivier, J. G., ... &
Minx, J. (2021). A review of trends and drivers of greenhouse gas emissions by sector from 1990 to 2018. Environmental research letters, 16(7), 073005.
Liao, H., Wei, Y., Ali, S., Uktamov, K. F., & Ali, N. (2023). Natural resources extraction
and industrial expansion: Natural resources a curse or blessing for the industrial sector of China?. Resources Policy, 85, 103986.
Ma, Z., Augustijn, K., De Esch, I., & Bossink, B. (2023). Public-private partnerships
influencing the initiation and duration of clinical trials for neglected tropical diseases. PLoS Neglected Tropical Diseases, 17(11), e0011760.
Mackey, T. K., Liang, B. A., Cuomo, R., Hafen, R., Brouwer, K. C., & Lee, D. E. (2014).
Emerging and reemerging neglected tropical diseases: a review of key characteristics, risk factors, and the policy and innovation environment. Clinical microbiology reviews, 27(4), 949-979.
Magalhães, A. R., Codeço, C. T., Svenning, J. C., Escobar, L. E., Van de Vuurst, P., &
Gonçalves-Souza, T. (2023). Neglected tropical diseases risk correlates with poverty and early ecosystem destruction. Infectious Diseases of Poverty, 12(1), 32.
Makam, P., & Matsa, R. (2021). “Big Three” infectious diseases: tuberculosis, malaria
and HIV/AIDS. Current Topics in Medicinal Chemistry, 21(31), 2779-2799.
Malecela, M. N. (2019). Reflections on the decade of the neglected tropical diseases.
International health, 11(5), 338-340.
Malecela, M. N., & Ducker, C. (2021). A road map for neglected tropical diseases
2021–2030. Transactions of the Royal Society of Tropical Medicine and Hygiene, 115(2), 121-123.
Manyeh, A. K., Ibisomi, L., Ramaswamy, R., Baiden, F., & Chirwa, T. (2020). Exploring
factors affecting quality implementation of lymphatic filariasis mass drug administration in Bole and Central Gonja Districts in Northern Ghana. PLoS neglected tropical diseases, 14(8), e0007009.
Martins-Melo, F. R., Carneiro, M., Ramos Jr, A. N., Heukelbach, J., Ribeiro, A. L. P., &
Werneck, G. L. (2018). The burden of neglected tropical diseases in Brazil, 1990-2016: a subnational analysis from the Global Burden of Disease Study 2016. PLoS neglected tropical diseases, 12(6), e0006559.
McDonald, R. A., Wilson-Aggarwal, J. K., Swan, G. J., Goodwin, C. E., Moundai, T.,
Sankara, D., ... & Zingeser, J. A. (2020). Ecology of domestic dogs Canis familiaris as an emerging reservoir of Guinea worm Dracunculus medinensis infection. PLoS neglected tropical diseases, 14(4), e0008170.
Molyneux, D. H. (2012). The'Neglected Tropical Diseases': now a brand identity;
responsibilities, context and promise. Parasites & Vectors, 5, 1-4.
Molyneux, D. H., Asamoa-Bah, A., Fenwick, A., Savioli, L., & Hotez, P. (2021). The
history of the neglected tropical disease movement. Transactions of the Royal Society of Tropical Medicine and Hygiene, 115(2), 169-175.
Molyneux, D., & Sankara, D. P. (2017). Guinea worm eradication: Progress and
challenges—should we beware of the dog?. PLoS Neglected Tropical Diseases, 11(4), e0005495.
Montresor, A., Gabrielli, A. F., Chitsulo, L., Ichimori, K., Mariotti, S., Engels, D., &
Savioli, L. (2012). Preventive chemotherapy and the fight against neglected tropical diseases. Expert review of anti-infective therapy, 10(2), 237-242.
More countries eliminate neglected tropical diseases but investments key to sustain
progress. (n.d.). Www.who.int. https://www.who.int/news/item/30-01-2023-more countries-eliminate-neglected-tropical-diseases-but-investments-key-to-sustain-progress
Mrazek, M. F., & Mossialos, E. (2003). Stimulating pharmaceutical research and
development for neglected diseases. Health policy, 64(1), 75-88.
Munoz, V., Visentin, F., Foray, D., & Gaule, P. (2015). Can medical products be
developed on a non-profit basis? Exploring product development partnerships for neglected diseases. Science and Public Policy, 42(3), 315-338.
Moshi, V. (2021). Implementation challenges of community directed treatment with
ivermectin program for control of onchocerciasis in ulanga, Tanzania. East African Health Research Journal, 5(2), 123-128.
Martín Nieto, R. (2007). Web Communication by ngdos. Signo y Pensamiento, (51),
130-136.
NDi, D. (2014). An innovative approach to R&D for neglected patients: Ten years of
experience and lessons learned by DNDi. DNDi.
Ochola, E. A., Karanja, D. M., & Elliott, S. J. (2021). The impact of Neglected Tropical
Diseases (NTDs) on health and wellbeing in sub-Saharan Africa (SSA): A case study of Kenya. PLOS Neglected tropical diseases, 15(2), e0009131.
Oliveira, R. G. D. (2018). Sentidos das Doenças Negligenciadas na agenda da Saúde
Global: o lugar de populações e territórios. Ciência & Saúde Coletiva, 23(7), 2291-2302.
Olliaro, P. L., Vaillant, M., Hayes, D. J., Montresor, A., & Chitsulo, L. (2013). Practical
dosing of praziquantel for schistosomiasis in preschool‐aged children. Tropical Medicine & International Health, 18(9), 1085-1089.
Onifade, A. A., Odeniran, P. O., Ademola, I. O., Yusuf, A., & Musa, S. S. (2024).
Modeling the dynamics of Onchocerca volvulus with the impact of environmental factors on blackfly breeding sites. Scientific African, 25, e02272.
Pan American Health Organization. (2022, January 28). SDG 3 - Target 3.3 -
PAHO/WHO | Pan American Health Organization. Paho.org.
https://www.paho.org/en/sdg-3-target-3-3
Phillips, M. C., LaRocque, R. C., & Thompson, G. R. (2024). Infectious diseases in a
changing climate. JAMA, 331(15), 1318-1319.
Pinto, B. R. (2019). Alocação de leitos de internação com o uso da abordagem de
otimização via simulação
Qian, M. B., Chen, J., & Zhou, X. N. (2020). Beating neglected tropical diseases: For
good and for all. China CDC Weekly, 2(6), 92.
Relman, D. A., & Choffnes, E. R. (Eds.). (2011). The causes and impacts of neglected
tropical and zoonotic diseases: opportunities for integrated intervention strategies. National Academies Press.
Ryan, S. J., Carlson, C. J., Mordecai, E. A., & Johnson, L. R. (2019). Global expansion
and redistribution of Aedes-borne virus transmission risk with climate change. PLoS neglected tropical diseases, 13(3), e0007213.
Savioli, L., Daumiere, D., & Savioli, D. D. (2012). Accelerating work to overcome the
global impact of neglected tropical diseases: a roadmap for implementation. Geneva: World Health Organization, 1-42.
Schmidt, C. A., Cromwell, E. A., Hill, E., Donkers, K. M., Schipp, M. F., Johnson, K. B.,
... & Hay, S. I. (2022). The prevalence of onchocerciasis in Africa and Yemen, 2000–2018: a geospatial analysis. BMC medicine, 20(1), 293.
Sightsavers. (2017). Elimination of onchocerciasis
Smith, J., & Taylor, E. M. (2013). MDGs and NTDs: reshaping the global health agenda.
PLoS neglected tropical diseases, 7(12), e2529.
Souza, A. A., Ducker, C., Argaw, D., King, J. D., Solomon, A. W., Biamonte, M. A., ... &
Lammie, P. J. (2021). Diagnostics and the neglected tropical diseases roadmap: setting the agenda for 2030. Transactions of The Royal Society of Tropical Medicine and Hygiene, 115(2), 129-135.
Steele, R. (Ed.). (2019). Progress on household drinking water, sanitation and hygiene
2000-2017: special focus on inequalities.
Tidman, R., Abela-Ridder, B., & de Castañeda, R. R. (2021). The impact of climate
change on neglected tropical diseases: a systematic review. Transactions of The Royal Society of Tropical Medicine and Hygiene, 115(2), 147-168.
United Nations. (2015). Transforming our world: The 2030 Agenda for Sustainable
Development. United Nations. https://sdgs.un.org/2030agenda
United Nations . (n.d.). World population prospects 2024: Summary of Results.
United Nations . (2015). Transforming our world: the 2030 Agenda for Sustainable
Development.
Uniting to Combat Neglected Tropical Diseases. (n.d.-a). Kigali Declaration on
Neglected Tropical Diseases.
Uniting to Combat Neglected Tropical Diseases. (n.d.-b). London Declaration on
Neglected Tropical Diseases.
Uniting to Combat Neglected Tropical Diseases. (2022). Kigali Declaration.
Uniting to Combat Neglected Tropical Diseases . (2025). Statement from Uniting to
Combat NTDs in support of US Government Neglected Tropical Disease Programmes. Uniting to Combat NTDs. https://unitingtocombatntds.org/en/news-and views/statement-from-uniting-to-combat-ntds-in-support-of-us-government-neglected tropical-disease-programmes/
Vanderslott, S. (2019). Moving from outsider to insider status through metrics: the i
inclusion of “neglected tropical diseases” into the sustainable development goals. Journal of Human Development and Capabilities, 20(4), 418-435.
Weng, H. B., Chen, H. X., & Wang, M. W. (2018). Innovation in neglected tropical
disease drug discovery and development. Infectious diseases of poverty, 7(03), 1-9.
Williams, K., Bilsland, E., Sparkes, A., Aubrey, W., Young, M., Soldatova, L. N., ... &
King, R. D. (2015). Cheaper faster drug development validated by the repositioning of drugs against neglected tropical diseases. Journal of the Royal society Interface, 12(104), 20141289.
World Bank . (1991). Onchocerciasis Control Programme [OCP] - Donors - Letters and Telex Sent to All.
https://thedocs.worldbank.org/en/doc/752941619424078141-0240021991/original/WorldBankGroupArchivesFolder30303452.pdf
World Health Organization. (n.d.-a). . Intensified control of neglected diseases: report of
an international workshop.
World Health Organization. (n.d.-b). An R&D Blueprint for Neglected Tropical Diseases.
World Health Organization. (n.d.-c). Neglected Tropical Diseases -- GLOBAL.
www.who.int. https://www.who.int/health-topics/neglected-tropical-diseases
World Health Organization. (2009). Methods for surveillance of antimalarial drug
efficacy. In Methods for surveillance of antimalarial drug efficacy.
World Health Organization. (2015). African programme for onchocerciasis control:
progress report, 2014–2015. Wkly Epidemiol Rec, 90(49), 661-74.
World Health Organization. (2019). Report of the 20th meeting of the WHO alliance for t
the global elimination of trachoma by 2020, Sydney, Australia, 26–28 April 2016. In Report of the 20th meeting of the WHO alliance for the global elimination of trachoma by 2020, Sydney, Australia, 26–28 April 2016.
World Health Organization. (2017). African Programme For Onchocerciasis Control;
World Health Organization. (2018). The expanded special project for elimination of
neglected tropical diseases (ESPEN) 2017 annual report.
World Health Organization. (2020). Ending the neglect to attain the Sustainable
Development Goals: a road map for neglected tropical diseases 2021–2030. In Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030.
World Health Organization. (2022a, January 10). Dracunculiasis (guinea-worm disease).
www.who.int. https://www.who.int/news-room/fact
sheets/detail/dracunculiasis-(guinea-worm-disease)
World Health Organization. (2022b, November 23). Onchocerciasis. Who.int; World
Health Organization: WHO. https://www.who.int/news-room/fact
sheets/detail/onchocerciasis
World Health Organization. (2023). Global report on neglected tropical diseases 2023.
World Health Organization.
World Health Organization. (2023). World malaria report 2023. World Health
Organization.
World Health Organization - Global Health Observatory. (2023). Reported number of
people requiring interventions against NTDs. Www.who.int. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/reported-number-of-people-requiring-interventions-against-ntds
Zhang, C., Zeng, G., Huang, D., Lai, C., Chen, M., Cheng, M., ... & Wang, R. (2019).
Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chemical Engineering Journal, 373, 902-922.



.jpg)
.jpg)
.jpg)
.jpg)






.svg)



.svg)



